To characterize, classify, prioritize and subsitute PFAS compounds
Reduce duration, financial and ecological costs, and fill existing information gaps by using digital innovations and artificial intelligence.

Preludéa®

What is Preludéa ?
Preludéa® is a platform that responds to the challenges of per- and polyfluoroalkyl substances (PFAS), a family of chemicals essential to our economy but known for their toxicity and persistence in the environment.
To face PFAS drawbacks, Harmonic Pharma introduces the Preludéa® platform, which incorporates a new alternative method for classifying, comparing, ranking and substituting PFAS.

How it works ?
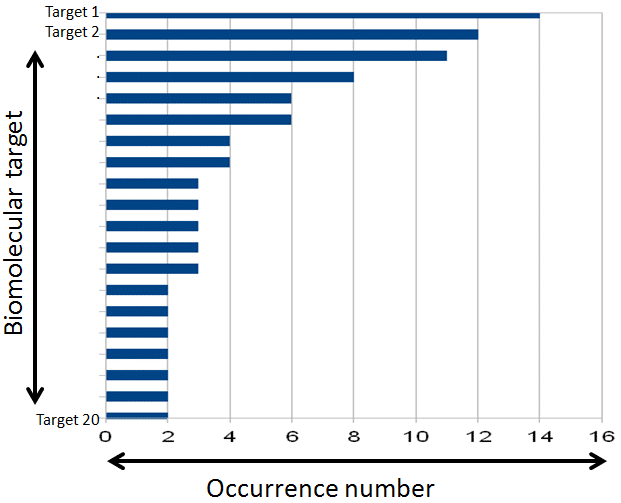
The innovative solution is based on OECD compliant toxicity predictions coupled with artificial intelligence, target analysis and spherical harmonics, 3D digital descriptors from which the company takes its name.

Who is concerned ?
Preludéa® is offered to professionals: engineers, researchers, senior technicians, executives, product managers, service managers, toxicologists and chemists from the various departments affected, such as those of Innovation, QSE, CSR, management, quality, Research and Development willing to acquire new skills, to discover and to find out insights for remediation stategy.
Preludéa® Solutions
Preludéa® is offered to professionals: engineers, researchers, senior technicians, executives, product managers, service managers, toxicologists and chemists from the various departments affected, such as those of Innovation, QSE, CSR, management, quality, Research and Development willing to acquire new skills, to discover and to find out insights for remediation stategy.
Service
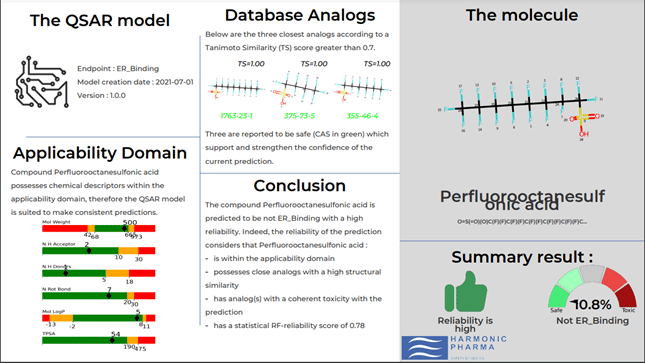
A method based on OECD-compliant toxicity predictions

Training
Preludéa® includes dedicated training



Service
This method gives the opportunity to discriminate the most problematic molecules and prioritize families of molecules that need to be monitored more specifically because of the lack of experimental data.
In addition, Preludéa® allows the exploration and evaluation of the possibility of substituting problematic PFAS compounds.
Software
The software allows the classification, comparison and overall evaluation of PFAS compounds on a large scale even in the absence of experimental data.
Please visit our dedicated page for more information.
Training
At the end of the two days training session, learners will have learned:
- Knowledge on the fundamentals of PFAS;
- The ability to master a specialized solution (Safety by Design®);
- The ability to assess PFAS issues and try to implement corrective measures.
Chronology of PFAS regulation
Per- and polyfluoroalkyl substances (PFAS) are a large family of several thousand synthetic chemicals that are commonly used throughout society and found in the environment. PFAS, also known as “Forever Chemicals”, are compounds containing a very stable “Carbon-Fluorine” bond. PFAS do not degrade after use or discharge into the environment because of this bond, which is one of the strongest in organic chemistry.